ST2 in detail
ST2 (for growth STimulation expressed gene 2) is a member of the interleukin-1 (IL1) receptor family of proteins, which play a central role in the regulation of immune and inflammatory response. Also referred to as cytokines, because of their cell-signaling capacity, the IL1 family was largely studied to elucidate the link between fever and infection or inflammation. Since initial elucidation, its diverse physiologic role now includes the stimulation and inhibition of cells in organs including the heart. In the heart, ST2 has a biological role in the innate immunological process and is also directly involved in a cardiac signaling pathway, which, under healthy conditions, serves to protect the heart during pressure overload or stretch (1).
The two key isoforms of ST2 are ST2L (a membrane-bound receptor) and sST2 (a soluble form found in the bloodstream). The response of healthy cardiac tissue to injury or mechanical stress involves the production and binding of interleukin-33 (IL-33) to ST2L, which stimulates a cardioprotective signaling cascade that defends against fibrosis, stiffening of the heart (cardiac remodeling), and heart failure (HF)(2). Heart failure is a progressive disease, which requires ongoing treatment. When sST2 levels are elevated, however, sST2 will bind to IL-33, thus reducing the beneficial effect of IL-33 through the ST2L receptor, so that cardiac fibrosis starts to develop. In this way sST2 is a biomarker for worse prognosis in patients with heart failure.
While all individuals have a normal level of ST2 in their circulation, an elevated concentration of ST2 is a powerful predictor of adverse outcomes, mortality or hospitalization, not only in patients with heart failure as well as other forms of cardiac disease, but also in the general population. The median normal concentration for ST2 is 18 ng/ml, while concentrations greater than 35 ng/ml are strongly indicative of increased risk (1-4). ST2 concentration elevation precedes an overt change in a patient’s symptoms, reflecting a worsening in the patient’s disease status. Plasma ST2 thus predicts which chronic HF patients are progressing towards worsening HF and cardiac remodeling, so that treatment can be implemented to improve the risk profile (2).
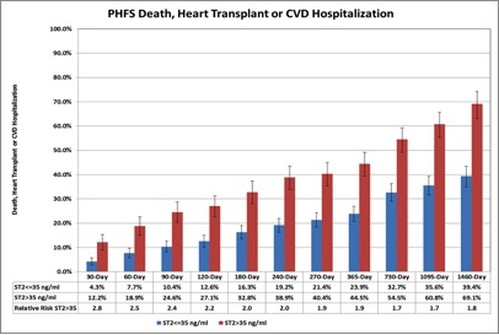
The Penn Heart Failure Study (PHFS) illustrates the significance of a high ST2 value for predicting clinically relevant outcomes, death, heart transplant or hospitalization in patients with HF (Figure 1). Patients with ST2 >35 ng/ml have a 2.8x higher risk of adverse outcomes within 30 days than patients with low ST2 concentrations. The relative risk of adverse events in patients with ST2 >35 ng/ml persists at a level of at least 1.8 for a follow up period of 4 years (4).
The two key isoforms of ST2 are ST2L (a membrane-bound receptor) and sST2 (a soluble form found in the bloodstream). The response of healthy cardiac tissue to injury or mechanical stress involves the production and binding of interleukin-33 (IL-33) to ST2L, which stimulates a cardioprotective signaling cascade that defends against fibrosis, stiffening of the heart (cardiac remodeling), and heart failure (HF)(2). Heart failure is a progressive disease, which requires ongoing treatment. When sST2 levels are elevated, however, sST2 will bind to IL-33, thus reducing the beneficial effect of IL-33 through the ST2L receptor, so that cardiac fibrosis starts to develop. In this way sST2 is a biomarker for worse prognosis in patients with heart failure.
While all individuals have a normal level of ST2 in their circulation, an elevated concentration of ST2 is a powerful predictor of adverse outcomes, mortality or hospitalization, not only in patients with heart failure as well as other forms of cardiac disease, but also in the general population. The median normal concentration for ST2 is 18 ng/ml, while concentrations greater than 35 ng/ml are strongly indicative of increased risk (1-4). ST2 concentration elevation precedes an overt change in a patient’s symptoms, reflecting a worsening in the patient’s disease status. Plasma ST2 thus predicts which chronic HF patients are progressing towards worsening HF and cardiac remodeling, so that treatment can be implemented to improve the risk profile (2).
The Penn Heart Failure Study (PHFS) illustrates the significance of a high ST2 value for predicting clinically relevant outcomes, death, heart transplant or hospitalization in patients with HF (Figure 1). Patients with ST2 >35 ng/ml have a 2.8x higher risk of adverse outcomes within 30 days than patients with low ST2 concentrations. The relative risk of adverse events in patients with ST2 >35 ng/ml persists at a level of at least 1.8 for a follow up period of 4 years (4).
Figure 1. Effect of high ST2 levels on adverse clinical outcomes in the Penn Heart Failure study (Chronic heart failure cohort, adapted from reference 4).
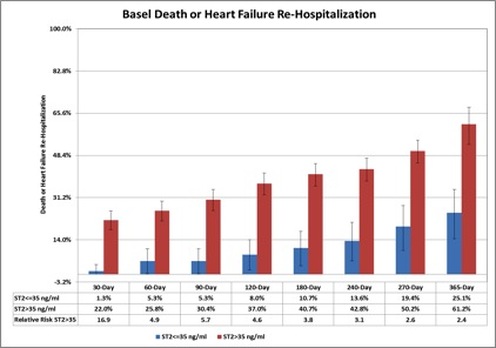
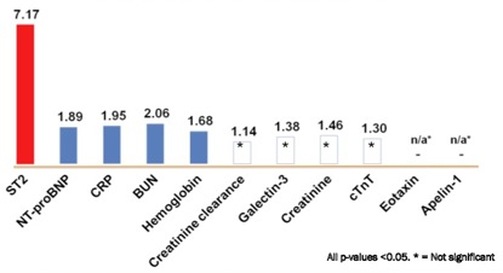
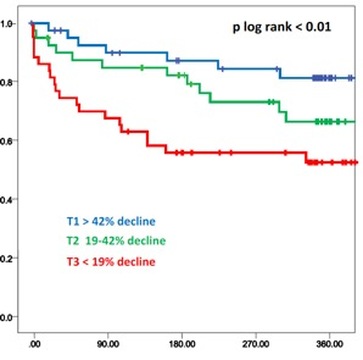
In the Basel (Switzerland) Heart Failure Study of patients admitted with acute heart failure, an elevated ST2 levels was a powerful predictor of an adverse clinical events within 1 year (figure 2)(5). An elevated ST2 was found to be particularly predictive of a 30-day adverse clinical event in patients with acute heart failure (RR=16.9, figure 2). In a similar US study, an elevated ST2 level was the most predictive biomarker for mortality within 1 year for patients admitted with acute heart failure (Pro-BNP Investigation of Dyspnea in the Emergency department study, figure 3)(6). Furthermore, early ST2 changes in patients admitted with acute heart failure was shown to be an independent predictor of 1 year mortality (figure 4)(7).
Figure 2. Effect of high ST2 levels on adverse clinical outcomes in the Basel Heart Failure study (acute heart failure cohort, adapted from reference 5).
Figure 3. ST2 is the most predictive biomarker for mortality within 1 year for patients admitted with acute heart failure in the PRIDE study (the numbers represent Hazard Ratio, adapted from reference 6).
Figure 4: Kaplan-Meier curve of patients stratified by change in early ST2 levels. In Cox-regression analysis, early ST2 changes independently predicted one-year mortality (adapted from reference 7).
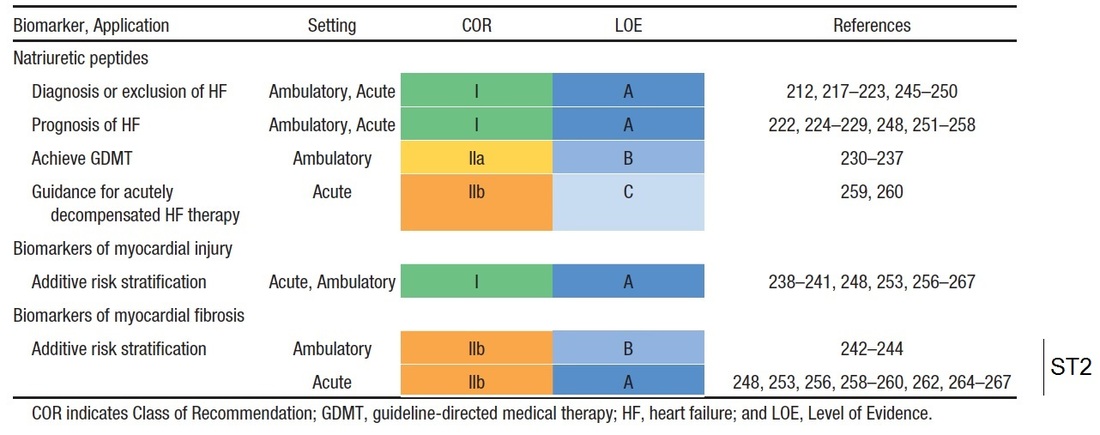
ST2 in the Heart Failure Guidelines
The role of ST2 as an useful additive risk stratification biomarker to the currently used BNP or NT-proBNP has been highlighted in the 2013 American College of Cardiology Foundation (ACCF) / American Heart Association (AHA) guideline for the management of heart failure (3).
Here, ST2 (a biomarker of myocardial fibrosis) has been described as not only predictive of hospitalisation and death in patients with heart failure, but it was additive to the natriuretic peptide levels in its prognostic value. As a result of this, ST2 was given the highest level of evidence of "A" especially in the context of risk stratification in patients with acute heart failure.
ST2 in practice
By measuring ST2 as part of a patient management program, clinicians can be more precise in caring for people with heart failure. This may lead to halting or slowing down the progression of the disease. While natriuretic peptide markers like BNP and NT-proBNP may help a physician diagnose heart failure in a symptomatic patient, in study after study, ST2 has consistently demonstrated improved accuracy of patient prognosis over peptides markers alone. ST2 also has twice the predictive value of Galectin-3.
Moreover, ST2 levels are not adversely affected by such confounding factors as age, gender, body mass index, atrial fibrillation, history of heart failure, anemia and impaired renal failure. And unlike natriuretic peptide markers and Galectin-3, ST2 has a single cutpoint, removing any guesswork, making treatment decisions easier.
Moreover, ST2 levels are not adversely affected by such confounding factors as age, gender, body mass index, atrial fibrillation, history of heart failure, anemia and impaired renal failure. And unlike natriuretic peptide markers and Galectin-3, ST2 has a single cutpoint, removing any guesswork, making treatment decisions easier.
References
1. Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Disc 2008; 7(10):827-840
2. Januzzi JL Jr. ST2 as a Cardiovascular Risk Biomarker: From the Bench to the Bedside. J Cardiovasc Transl Res 2013 6(4):493-500
3. Yancy CW et al. ACCF/AHA Guideline for the Management of Heart Failure, J Am Coll Cardiol 2013, doi: 10.1016/j.jacc.2013.05.019
4. Ky B et al. High sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure. Circ Heart Fail 2011; 4(2):180-7.
5. Socrates et al. Interleukin family member ST2 and mortality in acute dyspnoea. J Int Med 2010; 268:493-500
6. Rehman et al. Independent and incremental prognostic value of multimarker testing in acute dyspnea: Results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clinica Chimica Acta 2008; 392:41-45
7. Breidthardt et al. Heart Failure induced early ST2 changes may offer long-term therapy guidance. J Card Fail 2013; 19(12):821-8
1. Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Disc 2008; 7(10):827-840
2. Januzzi JL Jr. ST2 as a Cardiovascular Risk Biomarker: From the Bench to the Bedside. J Cardiovasc Transl Res 2013 6(4):493-500
3. Yancy CW et al. ACCF/AHA Guideline for the Management of Heart Failure, J Am Coll Cardiol 2013, doi: 10.1016/j.jacc.2013.05.019
4. Ky B et al. High sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure. Circ Heart Fail 2011; 4(2):180-7.
5. Socrates et al. Interleukin family member ST2 and mortality in acute dyspnoea. J Int Med 2010; 268:493-500
6. Rehman et al. Independent and incremental prognostic value of multimarker testing in acute dyspnea: Results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clinica Chimica Acta 2008; 392:41-45
7. Breidthardt et al. Heart Failure induced early ST2 changes may offer long-term therapy guidance. J Card Fail 2013; 19(12):821-8